A polar molecule has an uneven distribution of charges due to differences in electronegativity, resulting in a molecular dipole moment.
Introduction to Polar Molecules
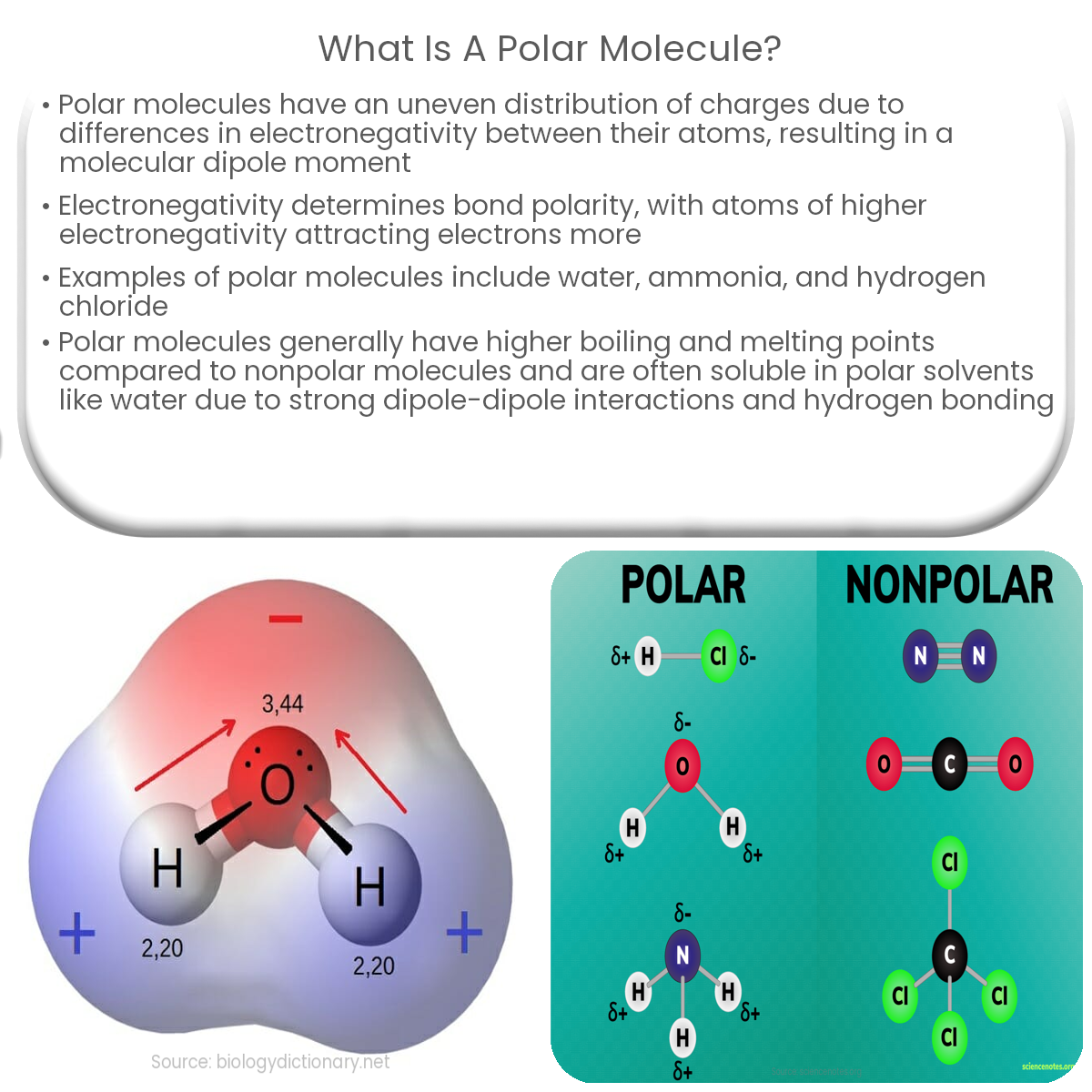
A polar molecule is a type of molecule that has an uneven distribution of charges due to the difference in electronegativity between the atoms involved. This results in the molecule having a partial positive charge on one side and a partial negative charge on the other side, creating a molecular dipole moment.
Electronegativity and Bond Polarity
Electronegativity is a measure of an atom’s ability to attract electrons in a chemical bond. When two atoms with different electronegativities form a covalent bond, the electrons are shared unequally between the atoms. The atom with the higher electronegativity attracts the electrons more, causing the bond to be polar. The difference in electronegativity between the two atoms determines the polarity of the bond.
Dipole Moment
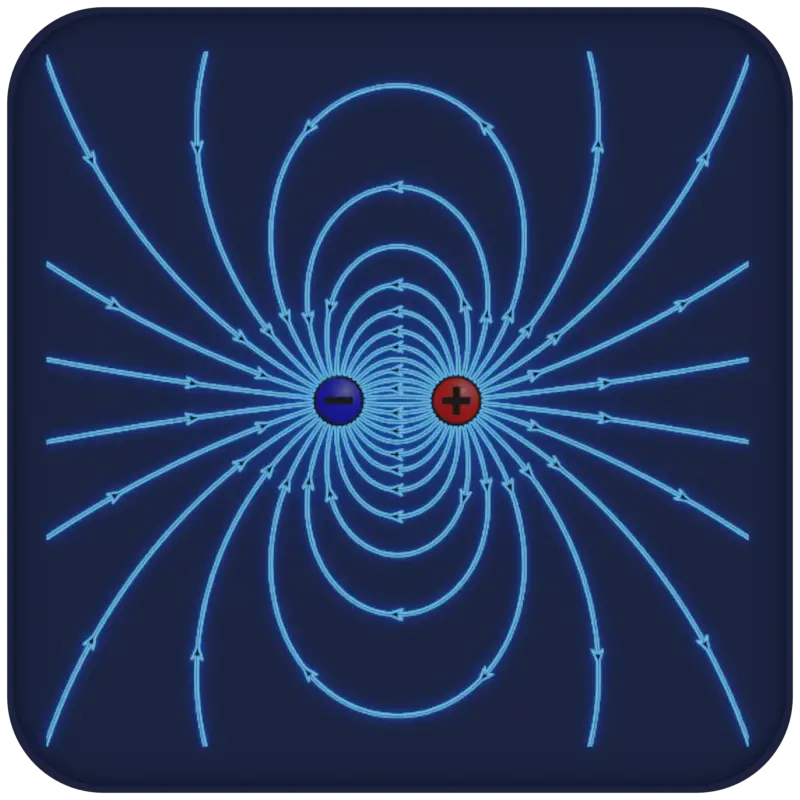
In polar molecules, the center of positive charge and the center of negative charge do not coincide, creating a molecular dipole moment. The magnitude of the dipole moment depends on the charges and the distance between them. Dipole moments are often represented by an arrow pointing from the positive to the negative end of the molecule, with the arrowhead indicating the direction of the negative charge.
Examples of Polar Molecules
- Water (H2O): In a water molecule, the oxygen atom is more electronegative than the hydrogen atoms, causing the electrons to be pulled towards the oxygen. This results in a bent molecular structure with a partial negative charge on the oxygen atom and partial positive charges on the hydrogen atoms.
- Ammonia (NH3): Ammonia consists of a central nitrogen atom bonded to three hydrogen atoms. Nitrogen has a higher electronegativity than hydrogen, leading to a molecular structure with a partial negative charge on the nitrogen atom and partial positive charges on the hydrogen atoms.
- Hydrogen chloride (HCl): In an HCl molecule, the electronegativity difference between hydrogen and chlorine causes the electrons to be attracted more towards the chlorine atom. This creates a polar bond with a partial negative charge on chlorine and a partial positive charge on hydrogen.
Properties of Polar Molecules
Polar molecules often have higher boiling and melting points compared to nonpolar molecules of similar size and structure. This is because the dipole-dipole interactions between polar molecules are stronger than the dispersion forces between nonpolar molecules. Additionally, polar molecules are often soluble in polar solvents, such as water, due to the ability of polar solvents to engage in dipole-dipole interactions and hydrogen bonding with polar solutes.